EasyNAT Malaria External Control
External Control Material for use with
Research Use Only Alethia Malaria DNA Amplification Assays
 Introduction
Introduction
The assay is intended for rapid qualitative detection of deoxyribonucleic
acid (DNA) of Malaria in human plasma, Venous blood, and fingertip blood specimens.
Malaria Assay EasyNAT®
Molecular Testing, Anywhere!
Accurate Safe
Simple
Fully closed test cartridge design.
No cross-contamination.
Fast
Get result within 49 minutes Easy workflow, no need for professional personnel or laboratory.
Catalog # U203010-20
EasyNAT® Malaria Assay
Venous blood; Plasma;
Fingertip blood
20 tests/kit
9 months
2~8°C
-25~30°C
EasyNAT® System
Test Item Malaria Cartridge ID Lot No.
Device NO. 012345
CoV54210202
Test Block 1 User USTAR
210202
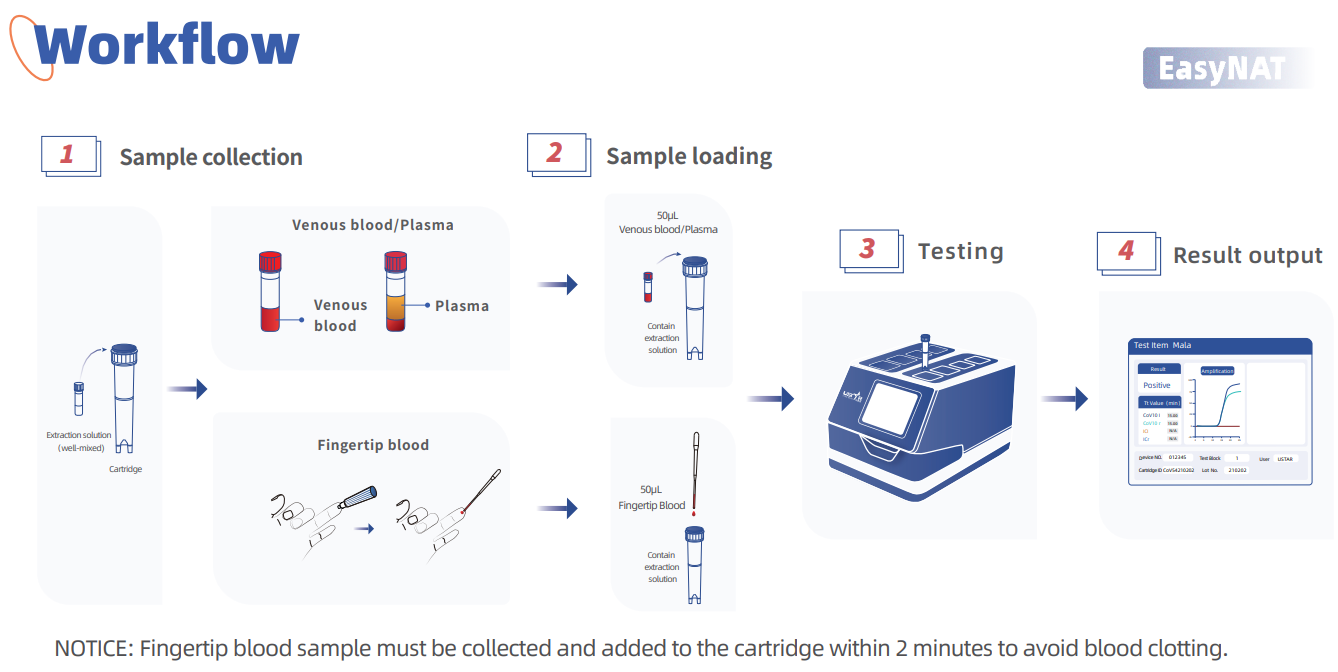
Workflow
Available for DNA from Plasmodium
Vivax, Malariae, Falciparum and Ovale.
Venous blood/Plasma Venous blood/Plasma
Fingertip blood
50μL
Venous
blood
Sample collection Sample loading
Cartridge
NOTICE: Fingertip blood sample must be collected and added to the cartridge within 2 minutes to avoid blood clotting.
50μL
Fingertip Blood

Similar to 479970RUO alethia Malaria External Control Kit or Alethia Malaria PLUS DNA Amplification
1. Malaria Positive Control: Tris-buffered solution with plasmid containing DNA inserts
(Plasmodium sp. and human mitochondrial DNA inserts) and azide (0.09%) as a preservative.
2. Malaria Negative Control: Tris-buffered solution with plasmid containing human mitochondrial
Compare with Ustar
DNA Amplification Test Kit, Meridian Bioscience, Inc. needs
1. Disposable latex gloves, powder free
2. DNAse/RNAse free, aerosol resistant pipette tips
EQUIPMENT NOT PROVIDED
1. Interval timer
2. Vortex Mixer (optional)
3. Micropipette capable of dispensing 50 µL
4. Micropipette capable of dispensing 250 µL (catalog number 481125RUO only)
5. Research Use Only Alethia Incubator/Reader™, Meridian Bioscience, Inc. Catalog Number:
PRECAUTIONS
1. All reagents are For Research Use Only. Not for use in diagnostic procedures.
2. This is a quality control reagent and is used to evaluate the performance of the RUO Alethia Malaria and
RUO Alethia Malaria PLUS DNA Amplification Assays. It is not directly used to test patient samples.
3. Do not eat, drink or smoke in areas where specimens or kit reagents are handled.
4. Wear disposable gloves while handling specimens and thoroughly wash hands afterwards.
5. Quality Control Programs for Molecular Testing Laboratories should be employed.
6. The RUO Alethia Malaria Test Devices contain lyophilized reagents. The protective pouch should not be
opened until ready to perform the assay.
7. The RUO Alethia Malaria Test Devices include a latch feature that is designed to prevent contamination of
the test area with amplification product. Do NOT use Test Devices with broken latches.
8. Dispose of used RUO Alethia Test Devices, M-prep Columns, and tubes immediately after processing,
leaving the device latch securely in place. Opening the device after amplification may result in contamination