Dengue IgG Elisa
Dengue IgG Capture ELISA
E-DEN02G
Please note these products are not for sale or distribution in the United States and may not be available in all countries worldwide. Please contact Inverness Medical or your local Panbio distributor to determine availability.
Product Overview
The Panbio Dengue IgG Capture ELISA is for the qualitative detection of IgG antibodies to dengue virus (serotypes 1, 2, 3 and 4) in serum. This test is intended as an aid in the clinical laboratory diagnosis of secondary dengue virus infection, and can be used in conjunction with the Panbio Dengue IgM Capture ELISA for the presumptive differentiation between primary and secondary infection.
The Panbio Dengue IgG Capture ELISA offers an alternative to the HAI assay as an aid in the serological diagnosis of secondary dengue infections.
The IgG cut-off is set to detect the high levels of IgG present in secondary infection.
As an aid in the diagnosis of secondary dengue
For use in endemic dengue regions
Use with the Dengue IgM Capture ELISA to detect and distinguish primary and secondary dengue with a high degree of confidence.
Quick Info
Excellent performance
Standardised method
Total incubation time 2 hr 10 min
Break-apart wells
Ready-to-use colour coded reagents
Compatible with standard microplate technology
Use in conjunction with E-DEN01M to differentiate primary and secondary infection
96 tests
Performance
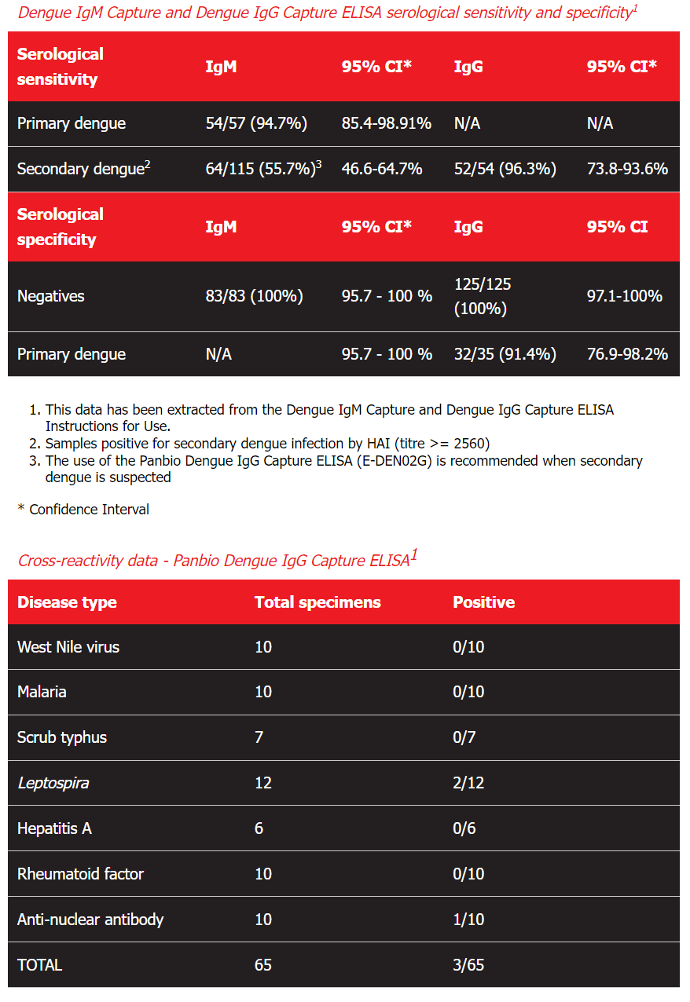
Dengue IgM Capture and Dengue IgG Capture ELISA serological sensitivity and specificity1
Serological sensitivity IgM 95% CI* IgG 95% CI*
Primary dengue
54/57 (94.7%)
85.4-98.91%
N/A
N/A
Secondary dengue2 64/115 (55.7%)3 46.6-64.7% 52/54 (96.3%) 73.8-93.6%
Serological specificity IgM 95% CI* IgG 95% CI
Negatives
83/83 (100%)
95.7 - 100 % 125/125 (100%) 97.1-100%
Primary dengue N/A 95.7 - 100 % 32/35 (91.4%) 76.9-98.2%
This data has been extracted from the Dengue IgM Capture and Dengue IgG Capture ELISA Instructions for Use.
Samples positive for secondary dengue infection by HAI (titre >= 2560)
The use of the Panbio Dengue IgG Capture ELISA (E-DEN02G) is recommended when secondary dengue is suspected
* Confidence Interval
Cross-reactivity data - Panbio Dengue IgG Capture ELISA1
Disease type Total specimens Positive
West Nile virus 10 0/10
Malaria 10 0/10
Scrub typhus 7 0/7
Leptospira 12 2/12
Hepatitis A 6 0/6
Rheumatoid factor 10 0/10
Anti-nuclear antibody 10 1/10
TOTAL 65 3/65
Publications
Cuzzubbo, A., et al. (1997). Commercial Assays for the Serological Diagnosis of Dengue Infection. Arbovirus Research in Australia. 7:56-60. Queensland Institute of Medical Research.
Cuzzubbo, A., et al (1998) Detection of Specific Antibodies in Saliva during Dengue Infection. Journal of Clinical Microbiology Dec. 1998:3737-3739
Cuzzubbo, A., et al. (1999) Comparison of PanBio Dengue Duo Enzyme-Linked Immunosorbent Assay (ELISA) and MRL Dengue Fever Virus Immunoglobulin M Capture ELISA for Diagnosis of Dengue Virus Infections in Southeast Asia. Clinical and Diagnostic Laboratory Immunology Sep. 1999:705-712
Lam, K., et al (2000) Evaluation of a Capture Screening Enzyme-Linked Immunosorbent Assay for Combined Determination of Immunoglobulin M and G Antibodies Produced during Dengue Infection. Clinical and Diagnostic Laboratory Immunology Sep. 2000:850-852
Porter, K., et al (1999) Evaluation of a Commercially Available Immunoglobulin M Capture Enzyme-Linked Immunosorbent Assay Kit for Diagnosing Acute Dengue Infections. Clinical and Diagnostic Laboratory Immunology Sep. 1999:741-744
Sang, C., et al (1998) Evaluation of a Commercial Capture Enzyme-Linked Immunosorbent Assay for Detection of Immunoglobulin M and G Antibodies Produced during Dengue Infection. Clinical and Diagnostic Laboratory Immunology Jan. 1998: 7-10
Vaughn, D., et al (1999) RAPID SEROLOGIC DIAGNOSIS OF DENGUE VIRUS INFECTION USING A COMMERCIAL CAPTURE ELISA THAT DISTINGUISHES PRIMARY AND SECONDARY INFECTIONS. Am. J. Trop. Med. Hyg. 60(4): 693-698
Vazquez, S., et al (2007) Evaluation of immunoglobulin M and G capture enzyme-linked immunosorbent assay Panbio kits for diagnostic dengue infections. Journal of Clinical Virology 39:194-198
Instructions for use